odparacetamol_single
Table of Contents
paracetamol overdose
see also:
introduction:
- this paper is a brief summary of the MJA 2019 consensus guidelines on Mx of paracetamol overdose to assist in efficient care.
- the far majority of paracetamol overdoses do not have any long term sequelae, however, some patients are at risk of developing potentially lethal hepatotoxicity over the succeeding few days due to a toxic metabolite which fortunately can be almost totally prevented if given IV N-acetylcysteine (NAC) within 8-12 hours of ingestion.
- a key factor may be a subsequent deficiency in hepatic MET (hepatocyte growth factor receptor) signaling - Hepatocyte growth factor (HGF), a pivotal mitogen for hepatocytes, is important for liver regeneration through activation of its receptor MET - MET is thought to play a critical protective role and is important for hepatic regeneration - it appears that MET is essential for both protecting and repairing the liver after a drug-induced overdose1)
- The N-acetyle cysteine (Parvolex) infusion is usually for 20 hours and thus if adult patients deemed safe from a security risk viewpoint, then consider admission to an ED Short Stay Observation Unit (SSU).
main roles of the ED doctor:
- determine timing and dose of overdose.
- should the patient be offered activated charcoal?
- does the patient need N-acetyle cysteine (Parvolex) and should it be started NOW instead of waiting on blood levels?
- could the patient have taken other dangerous medications in overdose concomitantly?
- is the patient safe for discharge from a medical viewpoint?
- is the patient safe for discharge from a mental health viewpoint (ie. what is the risk of suicide, etc)?
ED Mx of potential hepatotoxicity
indications for IV cannula and initial serum paracetamol and ALT
- all self-poisonings where paracetamol may have been accessed
- unintentional overdosages which may be at hepatotoxic levels (see below)
- the patient shows clinical signs suggestive of paracetamol hepatotoxicity (nausea, vomiting, right upper quadrant pain or tenderness) and paracetamol toxicity may be possible
- this should be done at least 4hrs post-ingestion
- add INR if > 24hrs since ingestion
- add U&E, LFTs, INR and VBG if referred in with an elevated ALT to assess pH and lactate
- at Western Health, as a minimum, order FBE, U&E, LFTs, glucose, serum paracetamol +/- HCG and others as indicated
indications for activated charcoal
- cooperative adult patients who have potentially ingested:
- immediate release paracetamol ≥ 10g or 200 mg/kg (whichever is less) within 2 hours, or,
- immediate release paracetamol ≥ 30 g within 4 hours post ingestion, or,
- modified-release paracetamol ≥ 10 g or ≥ 200 mg/kg (whichever is less) within 4 hours
- Note in larger overdoses activated charcoal may be indicated up to 24 hours post-ingestion.
- dose: adults 50g
- NB. activated charcoal is NOT indicated in children under 6 years of age, even if they might have ingested a toxic dose of paracetamol as significant hepatic injury is extremely rare in this group.
indications for commencing NAC immediately
- Treatment with acetylcysteine ensures survival if administered within 8 hours of paracetamol ingestion
- Beyond 8–10 hours after ingestion, efficacy decreases with increasing delay to treatment
- all those who ingest ≥ 10 g or 200 mg/kg (whichever is less) should immediately commence acetylcysteine and receive a full 20 hour course of acetylcysteine regardless of their serum paracetamol concentration(s).
- acute immediate release ingestions where serum levels will not be available within 8hrs of ingestion (or time unknown or more than 8hrs ago) and EITHER:
- ingestion is likely to be at hepatotoxic levels (see below)
- the patient shows clinical signs suggestive of paracetamol hepatotoxicity (nausea, vomiting, right upper quadrant pain or tenderness)
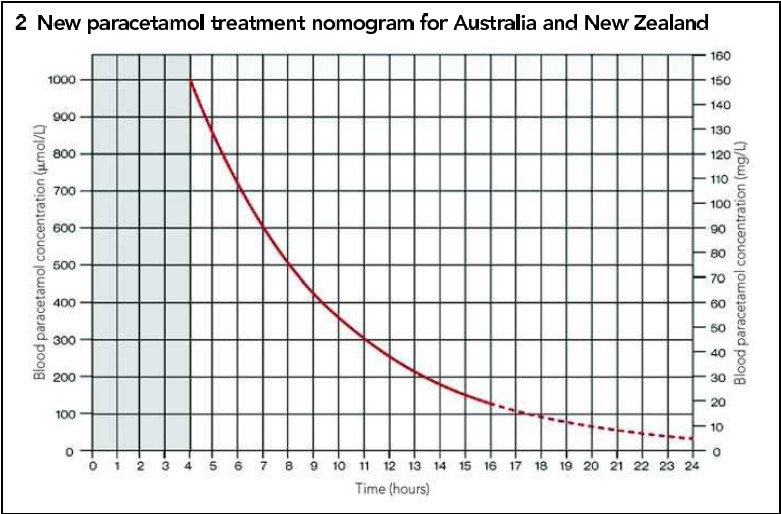
- serum paracetamol above the nomogram line (see below)
indications for referral to a liver transplant unit
- Consult the Liver Transplant Unit (or your local gastroenterology unit) if ANY of:
- INR > 3.0 at 48 hours or > 4.5 at any time
- oliguria or creatinine > 200 μmol/L,
- persistent acidosis (pH < 7.3) or arterial lactate > 3 mmol/L
- systolic hypotension with BP < 80mmHg, despite resuscitation
- hypoglycaemia, severe thrombocytopenia or encephalopathy of any degree,
- hepatic encephalopathy or any alteration of consciousness (GCS < 15) not associated with sedative co-ingestions.
- DO NOT GIVE clotting factors unless bleeding or after discussion with a Liver Transplant Unit
cessation of NAC infusion and medical clearance
- if full infusion regime indicated, a repeat serum paracetamol and ALT should be done just prior to the 20 hour infusion ending to ascertain whether an ongoing infusion is required
- NAC initial infusion regime can be ceased if:
- serum paracetamol > 4hrs post-ingestion under the nomogram AND serum ALT ≤ 50 U/L, OR,
- infusion regime completed and no ongoing infusion indicated (see below)
- Indications for ongoing infusion at the rate of the 2nd bag of NAC:
- Paracetamol concentration > 10 mg/L (66 μmol/L), OR
- ALT > 50 U/L and increasing (if baseline ALT > 50 U/L) BUT small fluctuations in ALT (e.g. +/- 20 U/L or +/-10%) are common and do not on their own indicate the need for ongoing acetylcysteine.
- Cease the ongoing infusion if ALL of the following apply:
- ALT or AST are decreasing
- INR < 2.0
- Patient clinically well
- for modified-release ingestions and those with an initial paracetamol concentration greater than double the nomogram line, paracetamol concentration has fallen below 10 mg/L (66 μmol/L)
Discharged patients
- Patients should be advised if they develop abdominal pain, nausea or vomiting further assessment is required.
patients at risk of hepatotoxicity
- MJA 2019 consensus guidelines suggest paracetamol may cause hepatoxicity if either:
- acute ingestion ≥ 10 g or ≥ 200 mg/kg (whichever is less), or,
- Repeated Supra-therapeutic Ingestion (RSTI):
- ≥ 10 g or ≥ 200 mg/kg (whichever is less) over a single 24-hour period, or,
- ≥ 12 g or ≥ 300 mg/kg (whichever is less) over a single 48-hour period, or,
- ≥ a daily therapeutic dose per day (for adults: total dose of 60 mg/kg over 24 hours and up to a maximum dose of 4 g/day) for more than 48 hours in those who also have abdominal pain or nausea or vomiting.
- staggered ingestions should be Mx as per acute single immediate release ingestions using the earliest time of ingestion
- thus if more than 8 hrs since the first ingestion, and suspected total dose is toxic, commence NAC infusion
- if serum paracetamol levels taken within 2 hours of the last dose, then repeat serum levels again in 2 hours and if either concentration is above the nomogram line (using time from the earliest ingestion), start/continue treatment with acetylcysteine
the treatment nomogram
- this nomogram image sourced from mja.com:
odparacetamol_single.txt · Last modified: 2026/02/03 00:08 by gary1